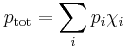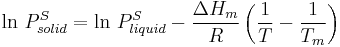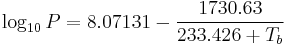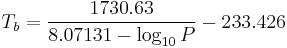Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form. Vice versa, all gases have a tendency to condense back to their liquid form, or deposit back to solid form, as long as the temperature is below their critical temperature or decomposition temperature. In plain terms, a liquid evaporates at all pressures below its vapor pressure, while remaining stable at pressure above the vapor pressure.
The equilibrium vapor pressure is an indication of a liquid's evaporation rate. It relates to the tendency of particles to escape from the liquid (or a solid). A substance with a high vapor pressure at normal temperatures is often referred to as volatile.
The vapor pressure of any substance increases non-linearly with temperature according to the Clausius–Clapeyron relation. The atmospheric pressure boiling point of a liquid (also known as the normal boiling point) is the temperature at which the vapor pressure equals the ambient atmospheric pressure. With any incremental increase in that temperature, the vapor pressure becomes sufficient to overcome atmospheric pressure and lift the liquid to form vapor bubbles inside the bulk of the substance. Bubble formation deeper in the liquid requires a higher pressure, and therefore higher temperature, because the fluid pressure increases above the atmospheric pressure as the depth increases.
The vapor pressure that a single component in a mixture contributes to the total pressure in the system is called partial pressure. For example, air at sea level, saturated with water vapor at 20 °C has a partial pressures of 23 mbar of water, and about 780 mbar of nitrogen, 210 mbar of oxygen and 9 mbar of argon.
Contents |
Measurement and units
Vapor pressure is measured in the standard units of pressure. The International System of Units (SI) recognizes pressure as a derived unit with the dimension of force per area and designates the pascal (Pa) as its standard unit. One pascal is one newton per square meter (N·m−2 or kg·m−1·s−2).
Experimental measurement of vapor pressure is a simple procedure for common pressures between 1 and 200 kPa.[1] Most accurate results are obtained near the boiling point of substances and large errors result for measurements smaller than 1kPa. Procedures often consist of purifying the test substance, isolating it in a container, evacuating any foreign gas, then measuring the equilibrium pressure of the gaseous phase of the substance in the container at different temperatures. Better accuracy is achieved when care is taken to ensure that the entire substance and its vapor are at the prescribed temperature. This is often done, as with the use of an isoteniscope, by submerging the containment area in a liquid bath.
Relation to boiling point of liquids
As a general trend, vapor pressures of liquids at ambient temperatures increase with decreasing boiling points. This is illustrated in the vapor pressure chart (see right) that shows graphs of the vapor pressures versus temperatures for a variety of liquids.[2]
For example, at any given temperature, propane has the highest vapor pressure of any of the liquids in the chart. It also has the lowest normal boiling point (−42.1 °C), which is where the vapor pressure curve of propane (the purple line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapor pressure.
Although the relation between vapor pressure and temperature is non-linear, the chart uses a logarithmic vertical axis to produce slightly curved lines, so one chart can graph many liquids. The vapor pressure of a liquid at its boiling point equals the pressure of its containing environment.
Liquid mixtures
Raoult's law gives an approximation to the vapor pressure of mixtures of liquids. It states that the activity (pressure or fugacity) of a single-phase mixture is equal to the mole-fraction-weighted sum of the components' vapor pressures:
where p is vapor pressure, i is a component index, and χ is a mole fraction of that component in liquid mixture. The term 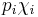 is the partial pressure of component i in the mixture. Raoult's Law is applicable only to non-electrolytes (uncharged species); it is most appropriate for non-polar molecules with only weak intermolecular attractions (such as London forces).
is the partial pressure of component i in the mixture. Raoult's Law is applicable only to non-electrolytes (uncharged species); it is most appropriate for non-polar molecules with only weak intermolecular attractions (such as London forces).
Systems that have vapor pressures higher than indicated by the above formula are said to have positive deviations. Such a deviation suggests weaker intermolecular attraction than in the pure components, so that the molecules can be thought of as being "held in" the liquid phase less strongly than in the pure liquid. An example is the azeotrope of approximately 95% ethanol and water. Because the azeotrope's vapor pressure is higher than predicted by Raoult's law, it boils at a temperature below that of either pure component.
There are also systems with negative deviations that have vapor pressures that are lower than expected. Such a deviation is evidence for stronger intermolecular attraction between the constituents of the mixture than exists in the pure components. Thus, the molecules are "held in" the liquid more strongly when a second molecule is present. An example is a mixture of trichloromethane (chloroform) and 2-propanone (acetone), which boils above the boiling point of either pure component.
Solids
Equilibrium vapor pressure can be defined as the pressure reached when a condensed phase is in equilibrium with its own vapor. In the case of an equilibrium solid, such as a crystal, this can be defined as the pressure when the rate of sublimation of a solid matches the rate of deposition of its vapor phase. For most solids this pressure is very low, but some notable exceptions are naphthalene, dry ice (the vapor pressure of dry ice is 5.73 MPa (831 psi, 56.5 atm) at 20 degrees Celsius, meaning it causes most sealed containers to explode), and ice. All solid materials have a vapor pressure. However, due to their often extremely low values, measurement can be rather difficult. Typical techniques include the use of thermogravimetry and gas transpiration.
The sublimation pressure can be calculated[3] from extrapolated liquid vapor pressures (of the supercooled liquid) if the heat of fusion is known. The heat of fusion must be added, in addition to the heat of vaporization to vaporize a solid. Assuming that the heat of fusion is temperature-independent and ignoring additional transition temperatures between different solid phases the equation
with:
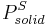 |
= Sublimation pressure of the solid component at the temperature 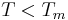 |
|---|---|
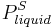 |
= Extrapolated vapor pressure of the liquid component at the temperature 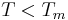 |
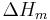 |
= Heat of fusion |
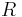 |
= Gas constant |
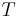 |
= Sublimation temperature |
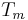 |
= Melting point temperature |
gives a fair estimation for temperatures not too far from the melting point. This equation also shows that the sublimation pressure is lower than the extrapolated liquid vapor pressure (ΔH m is positive) and the difference grows with increased distance from the melting point.
Boiling point of water in nature
Like all liquids, water boils when its vapor pressure reaches its surrounding pressure. In nature, the atmospheric pressure is lower at higher elevations and water boils at a lower temperature. The boiling temperature of water for atmospheric pressures can be approximated by Antoine equation:
or transformed into this temperature-explicit form:
where the temperature 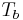 is the boiling point in degrees Celsius and the pressure
is the boiling point in degrees Celsius and the pressure 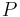 is in Torr.
is in Torr.
Dühring's rule
Dühring's rule states that a linear relationship exists between the temperatures at which two solutions exert the same vapor pressure.
Examples
The following table is a list of a variety of substances ordered by increasing vapor pressure.
| Substance | Vapor Pressure (SI units) |
Vapor Pressure (Bar); |
Vapor Pressure (mmHg); |
Temperature |
|---|---|---|---|---|
| Tungsten | 100 Pa | 0.001 | 0.75 | 3203 °C |
| Ethylene glycol | 500 Pa | 0.005 | 3.75 | 20 °C |
| Xenon difluoride | 600 Pa[4] | 0.006 | 4.50 | 25 °C |
| Water (H2O) | 2.3 kPa | 0.023 | 17.5 | 20 °C |
| Propanol | 2.4 kPa | 0.024 | 18.0 | 20 °C |
| Ethanol | 5.83 kPa[5] | 0.0583 | 43.7 | 20 °C |
| Methyl isobutyl ketone | 26.48 kPa | 0.2648 | 198.62 | 25 °C |
| Freon 113 | 37.9 kPa | 0.379 | 284 | 20 °C |
| Acetaldehyde | 98.7 kPa | 0.987 | 740 | 20 °C |
| Butane | 220 kPa | 2.2 | 1650 | 20 °C |
| Formaldehyde | 435.7 kPa | 4.357 | 3268 | 20 °C |
| Propane | 1.013 MPa | 10.133 | 7600 | 25.6 °C |
| Carbonyl sulfide | 1.255 MPa | 12.55 | 9412 | 25 °C |
| Carbon dioxide | 5.7 MPa | 57 | 42753 | 20 °C |
Meaning in meteorology
In meteorology, the term vapor pressure is used to mean the partial pressure of water vapor in the atmosphere, even if it is not in equilibrium,[6] and the equilibrium vapor pressure is specified otherwise. Meteorologists also use the term saturation vapor pressure to refer to the equilibrium vapor pressure of water or brine above a flat surface, to distinguish it from equilibrium vapor pressure, which takes into account the shape and size of water droplets and particulates in the atmosphere.[7]
See also
- Absolute humidity
- Clausius-Clapeyron relation
- Partial pressure
- Relative humidity
- Relative volatility
- Saturation vapor density
- Triple point
- Vapor-liquid equilibrium
- Vapor pressure of water
- Volatility
- Reid Vapor Pressure
- True vapor pressure
- Vapor pressures of the elements (data page)
References
- ^ K. Růžička, M. Fulem, V. Růžička. "Vapor Pressure of Organic Compounds. Measurement and Correlation". http://www.capec.kt.dtu.dk/documents/overview/Vapor-pressure-Ruzicka.pdf.
- ^ Perry, R.H. and Green, D.W. (Editors) (1997). Perry's Chemical Engineers' Handbook (7th Edition ed.). McGraw-Hill. ISBN 0-07-049841-5.
- ^ Moller B., Rarey J., Ramjugernath D., "Estimation of the vapour pressure of non-electrolyte organic compounds via group contributions and group interactions ", J.Mol.Liq., 143(1), 52-63, 2008
- ^ [1]
- ^ Saturated vapor pressure calculation at DDBST.com
- ^ Glossary (Developed by the American Meteorological Society)
- ^ A Brief Tutorial (An article about the definition of equilibrium vapor pressure)